Abstract
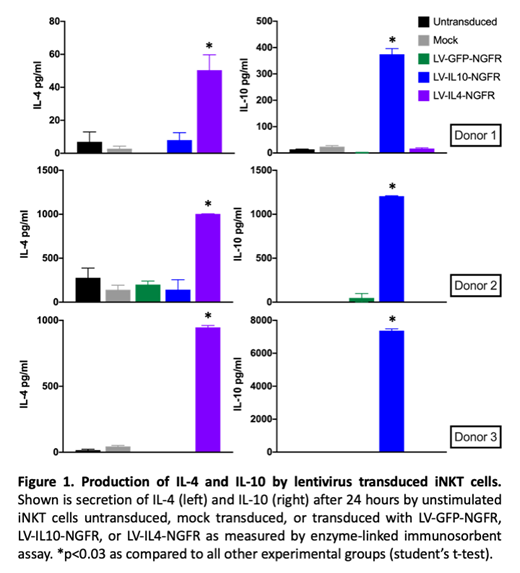
Many studies have shown the important role that invariant natural killer T (iNKT) cells play in suppression of graft-versus-host disease (GVHD). Murine studies have also demonstrated multiple subsets of iNKT cells, including those with pro-inflammatory and immunosuppressive properties. The prevention of murine acute GVHD by the adoptive transfer of immunomodulatory iNKT cells suggests that this approach would be ideal for clinical translation. However, the heterogeneity of human iNKT cells remains poorly understood, and the uncertain ability to identify immunosuppressive human iNKT cells and expand them to clinically relevant numbers remains a limitation in clinical translation. Therefore, engineering bulk human iNKT cells to enhance immunosuppressive capacity represents a novel approach to ensuring sufficient cell numbers of immunoregulatory cells for adoptive transfer. Herein, we describe the lentiviral transduction of human iNKT cells to overexpress immunomodulatory cytokines. We focused on IL-4, because murine iNKT cell-mediated GVHD suppression is IL-4 dependent, and IL-10, because an immunosuppressive regulatory T cell-like subset of murine iNKT cells highly produces IL-10. Human iNKT cells were transduced with a lentiviral construct expressing IL-4 or IL-10 (or green fluorescent protein (GFP) as a control) and an inert truncated nerve growth factor receptor (deltaNGFR) to facilitate sorting of transduced cells. Enzyme linked immunosorbent assay revealed significantly increased expression of IL-4 or IL-10 by cells transduced with the corresponding lentivirus, as compared to untransduced, mock transduced, or GFP transduced cells, replicated with 3 iNKT donors (p<0.03) (Figure 1). This proof-of-concept data demonstrates the ability to effectively transduce human iNKT cells to overexpress immunomodulatory cytokines. Future studies will focus on evaluating the immunosuppressive capacity of these engineered cells both in vitro and in vivo (using the xenograft GVHD model). Successful suppression of GVHD by engineered iNKT cells will provide a critical step forward in identifying novel approaches to bring these promising cells to the clinic.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal